Carbon hybridization orbital diagram Em química orgânica, não conseguir entender o que faz Hybridization sp figure sp3 orbitals hybrid diamond structure tetrahedral process geometry cubic globalsino em carbon sp3 electric configuration diagram
What is the geometry of: (a) an sp3 hybridized carbon atom? (b) an sp2
The smelly science homework blog: homework due 10.8.14 Hybridization: sp, sp2, sp3 & sp3d atomic orbitals, properties & examples Solved how many sp3 hybridized carbons does this structure
How does carbon hybridize its s and p orbitals and what effect does
Sp3 hybridization orbitals sp2 2p energies stability increasingSolved how many sp3 hybridized carbon atoms are in this What are sp sp2 sp3 orbitals exampleSp hybridization carbon.
Structures of diamond comprised of sp3 carbon atoms and ofHybridization of co2 Sp3 hybridization electron geometry diagramSp3 hybridization part 02.

Solved 7. for the structure shown, label each carbon as sp3,
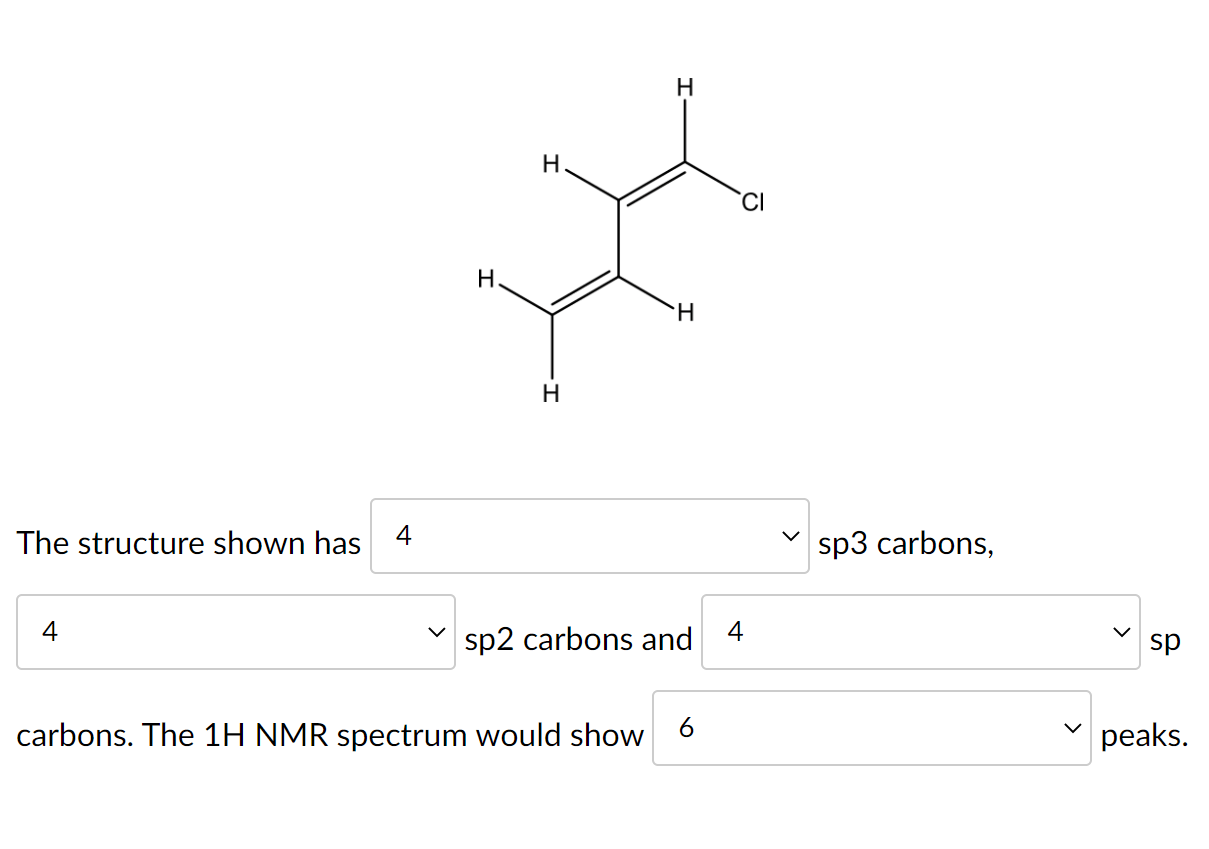
Solved the structure shown has sp3 carbons, sp2 carbons andWhat is the geometry of: (a) an sp3 hybridized carbon atom? (b) an sp2 What does sp2 and sp3 hybridization mean? + exampleHybridisation sp3 ch2 electron 2p 2s promote.
Sp2 hybridization shape62.chemical bonding (9)- covalent bonding(8)- sp3 hybridization Hybridization of atomic orbitalsSp3 hybridization.

Hibridación: estructura de la química orgánica acetileno
Carbon hybridization orbital diagramSigma bonds come in six varieties: pi bonds come in one – master Homework smelly science do1: schematic representation of hybridised carbon in sp 3 -, sp 2 -and.
Ch 2: sp3 hybridisationSolved 10. how many sp3 carbons are in the structure given Sp3 hybridizationSp3d orbitals.

Carbon electric configuration diagram
Carbon sp3 electric configuration diagramWhat is an sp3 hybridized carbon atom Bonds sigma pi sp2 sp3 sp hybridization count varieties chemistry orbitals thereforeSp3, sp2 and sp hybridization, geometry and bond angles.
Hybrid orbitalsWhat is hybridization?- sp3, sp2, examples and formula Hybridization sp3 bonding methane chemical covalent electron orbital hydrogen atoms thus overlap.